
Abbott, a company developing chronic pain therapy solutions, offers a variety of chronic pain relief devices, including spinal cord stimulation therapy, BurstDR stimulation, and dorsal root ganglion stimulation.
Although pain management doctors may sometimes recommend the Abbott Spinal Cord Stimulator for back and neck pain, there are numerous risks associated with the surgical procedure.
This article contains everything you need to know about the Abbott Spinal Cord Stimulator, including the disadvantages and risks of the surgery and whether or not it works to cure chronic back and neck pain.
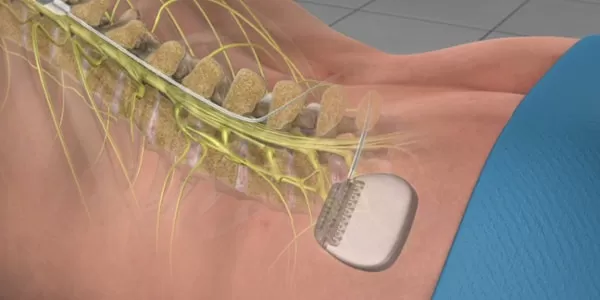
Abbott’s Spinal Cord Stimulator is a low-dose and low-energy pain management device designed to provide pain relief and improve system longevity. It works to relieve back pain by sending low-level electrical currents directly into the spinal cord. The FDA approved the company’s Proclaim XR recharge-free neurostimulation system for people living with chronic pain.
It’s possible to alter the device to save energy and battery life while maintaining pain alleviation. With this spinal cord stimulation system, the battery lasts up to 10 years at low-dose settings and doesn’t need to be charged.
Spinal cord stimulation involves the use of a spinal cord stimulator to deliver electrical impulses to the spinal cord to alleviate back or neck pain. The spinal cord stimulator is a battery-powered device that is implanted beneath the skin on the back.
The spinal cord stimulator consists of two components: the pulse generator and electrodes. The pulse generator is a compact device that generates electrical impulses to be sent to the spinal cord. It comprises a battery, a computer chip, and electrical leads that extend from the spinal cord stimulator.
The electrodes are a series of wires that convey the impulses to the spinal cord. These electrodes are positioned between the spinal cord and the vertebrae (the epidural space), and the generator is placed under the skin, typically near the abdomen or buttocks.
Spinal cord stimulators enable patients to control the electrical impulses using a remote control whenever they experience pain. Both the remote control and its antenna are external to the body.
Neuromodulation products are medical devices that use electrical or chemical stimulation to modulate the activity of the nervous system. They are used to treat a variety of conditions, including:
Neuromodulation devices can be implanted surgically or used non-invasively. Implanted devices typically consist of a pulse generator, which is implanted under the skin, and leads, which are placed near the nerves or area of the brain that is being targeted.
Non-invasive devices may use electrical stimulation, magnetic fields, or ultrasound to stimulate the nervous system. It’s important to note that neuromodulation is not a cure for any of the conditions it is used to treat, but it can be used to manage symptoms and improve quality of life.
The Abbott spinal cord stimulator is a device that’s been found to be more effective than traditional therapy at relieving chronic pain. It’s used when other traditional treatment plans for chronic pain–such as physical therapy, psychological interventions, nerve blocks, and surgery–haven’t worked.

Abbott’s spinal cord stimulation is most typically used to relieve pain in the legs, back, and arms that have not responded to surgery. It can also alleviate back pain in cases where spinal surgery is not an option.
Complex regional pain syndrome (CRPS), pain after nerve damage, refractory pain, post-herpetic neuralgia, and peripheral vascular disease are all illnesses that may respond to Abbott’s spinal cord stimulation.
The neurostimulator system is specifically designed to enhance patient outcomes by using the lowest effective dose of stimulation to provide pain relief and increase system longevity, giving patients a life-changing pain treatment choice without the hassles of recharging.
Thanks to a battery that lasts up to ten years at low-dose settings, you can enjoy pain treatment without charging the system. Abbott also incorporates Bluetooth wireless technology and familiar Apple mobile digital devices to help patients control pain.
Abbott’s low-energy spinal cord stimulation is a non-opioid solution offering an innovative approach to chronic pain management. The device works by changing pain signals from the spinal cord to the brain and with low dosages of mild electrical pulses.
This stimulation therapy entails surgically implanting a stimulator that targets the spinal cord to relieve pain in the lower limbs caused by complex regional pain syndrome. Individuals are given a hand-held iPod controller to modify the stimulation settings within certain prescribed limits.
The device was created in response to positive results from Abbott’s patients (BOLD) study, which discovered that 24 enrolled patients on a low-energy program experienced pain relief with less than six hours of battery use per day. Approximately half of those patients achieve pain relief with the lowest effective dose (less than two hours of battery use per day).
It has made a significant difference in treating chronic pain. The result: the Abbott spinal cord stimulation (SCS) helps extend the system’s battery life, allowing users to get pain relief for up to 10 years without worrying about recharging.
The long-term success rate of spinal cord stimulators (SCS) is currently between 47% and 74%. The duration between the onset of chronic pain syndrome and spinal cord stimulation implantation is inversely related to its effectiveness.
Therefore, implantation should be done as soon as possible to improve outcomes.
Despite the decent success rate, the Abbott spinal cord stimulator can cause various side effects, such as:
This device can be contraindicated for patients who are:
Therefore, it is critical to discuss any risks associated with the implant surgery and device with your doctor.
The cost of an Abbott Spinal Cord Stimulator, like other spinal cord stimulators, can vary widely based on several factors, including insurance coverage, medical necessity, and the specific model of the device. For individuals without insurance, the out-of-pocket costs for spinal cord stimulation can range from $15,000 to $50,000 or more. Insurance companies, including Medicare, often cover the cost of spinal cord stimulators if the treatment is deemed medically necessary.
Another source mentions that without insurance, one should expect to pay between $31,000 and more than $51,000 out-of-pocket for a spinal cord stimulator. Costs might vary based on the provider and the specific conditions set by insurance companies for coverage.
For example, Blue Cross Blue Shield clients might face implantation costs of around $60,000, while Medicare beneficiaries may have to pay almost $33,000, with annual fees between $6,000 and $22,000. The spinal cord stimulator device itself is estimated to cost around $20,000.
The following are some of the disadvantages of Abbott Spinal Cord Stimulators:
Some risks specific to Abbott’s spinal cord stimulator may include:
Abbott’s spinal cord stimulators, designed to alleviate chronic pain through neurostimulation, have faced technical and medical challenges. Notably, the FDA issued a Class I recall affecting over 155,000 Proclaim and Infinity devices due to a malfunction preventing devices from being turned back on after MRI scans, which led to the loss of therapy and additional surgeries for some patients.
Device-related complications, including lead migration, failed connections, and lead breakage, were reported in 38% of the study’s participants.
Patients have also experienced side effects like weakness, tingling in the legs, cerebrospinal fluid leakage, and more severe risks such as epidural bleeding and infection. Surgical risks are inherent to the implantation process, with infection and bleeding being primary concerns. While these devices offer significant pain management benefits, there are potential complications.
The U.S. Food and Drug Administration (FDA) has issued a Class I recall for Abbott’s Proclaim and Infinity implantable neurostimulators due to technical malfunctions related to the existing Magnetic Resonance Imaging (MRI) mode. This recall is classified as Class I, indicating that the use of these devices could result in serious injuries or death. The issue primarily involves patients being unable to turn the devices back on after an MRI, leading to a loss of therapy and, in some cases, necessitating additional surgery to replace the malfunctioning device. Despite the severity of the recall, patients are not currently being advised to have the devices surgically removed if they are not experiencing problems.
The recall affects over 155,000 units of the Proclaim and Infinity devices distributed between November 21, 2015, and June 29, 2023. The problem arises when the patient controller, an iPhone or iPod with a special application, cannot communicate with the implantable pulse generator (IPG) to exit MRI mode due to issues like software updates or deletion of the device from Bluetooth pairings. There have been 186 reported incidents and 73 injuries, though no deaths have been reported. Affected devices include various models of the Proclaim XR, Proclaim Plus, Proclaim DRG, and Infinity IPGs.
Abbott has taken steps to address the issue by sending an “urgent medical device correction” letter to healthcare providers in July, clarifying the instructions for exiting MRI mode and recommending not to delete the Bluetooth connection between the IPG and the patient controller during this process. Physicians are advised to ensure patients do not delete this connection and to keep the patient controller app up to date.
Implantable neurostimulators like those recalled are used as a last resort for treating chronic pain in the back, legs, or head by emitting low-level electrical impulses to block pain signals. Despite their intended benefit of reducing the need for opioids and other pain therapies, studies have indicated that many patients do not reduce their use of other pain management methods, and a significant number experience complications severe enough to require device removal or revision.
This recall has drawn attention to the safety record of spinal cord stimulators (SCSs), highlighting the need to carefully consider the risks and benefits of such devices.
The Abbott spinal cord stimulator lawsuit centers on allegations of defective products and negligence, with patients reporting adverse effects and complications. The lawsuit aims to hold Abbott accountable for alleged issues such as device failure, infections, and ineffective pain relief, which have led to further complications and distress for some patients.
Those affected may seek compensation for damages like medical expenses, pain, and emotional distress. Legal representation is advised for navigating the medical device lawsuit process.
The use of SCS devices, including those produced by Abbott, involves certain safety issues and potential adverse events that patients need to be aware of:
Also, while SCS devices offer significant pain relief for many patients, they do not cure the underlying causes of pain. The relief provided by SCS is due to the electrical impulses that interfere with the pain signals being sent to the brain, rather than resolving the condition causing the pain.
This means the primary function of SCS is to manage pain rather than to cure the underlying condition causing the pain. It’s a form of therapy that masks the pain signals before they reach the brain.
Also, the effectiveness of SCS varies from patient to patient. While many experience significant pain relief, others may find the therapy less effective. The success of SCS can depend on numerous factors, including the cause and location of the pain, as well as the patient’s overall health and response to the device.
The Cochrane Review, led by the University of Sydney, critically assessed the available clinical evidence regarding the use of SCS for chronic back pain and found significant limitations in its long-term effectiveness and safety profile.
According to the Cochrane Review, the analysis of 13 clinical trials involving 699 participants revealed no significant benefit of SCS over placebo or no treatment in improving low back pain or quality of life. This suggests that, while some individuals report temporary pain relief from SCS, the scientific evidence does not support sustained benefits that outweigh the potential costs and risks associated with the procedure.
The review showed a concerning lack of data on the long-term safety and effectiveness of SCS, with little to no evidence available beyond a six-month period post-implantation. This gap in knowledge raises questions about the justification of SCS as a treatment option for chronic back pain, given its invasive nature and the significant financial costs involved.
The potential risks associated with SCS, such as nerve damage, infection, and the need for repeated surgeries due to complications like lead movement, further complicate the decision to proceed with this treatment. The Cochrane Review’s findings suggest that the adverse effects of SCS surgery are not well-documented, making it difficult to fully understand the risk profile of this intervention.
The review calls for more rigorous, long-term research to better understand the benefits, risks, and cost-effectiveness of SCS compared to non-invasive treatment options. For individuals considering SCS, it is crucial to have a comprehensive discussion with healthcare professionals about the potential benefits and risks, taking into account the current evidence base and exploring alternative pain management strategies.
Luckily, there is an alternative solution to the spinal cord stimulator for chronic pain: Deuk Laser Disc Repair.
Deuk Spine Institute is the world leader in minimally invasive Laser Spine Surgery. Dr. Ara Deukmedjian, founder of Deuk Spine Institute, developed the world’s most advanced laser spine surgery. This laser spine surgery is known as Deuk Laser Disc Repair.
Deuk Laser Disc Repair is used as an alternative to dangerous invasive surgeries like total disc replacement and spinal fusion. Deuk Laser Disc Repair is a form of endoscopic spine surgery performed in a state-of-the-art outpatient surgery center under sedation while the patient relaxes.
This procedure does not compromise or weaken the health and integrity of the spine.
While the complication rate for lower back and basic spine surgery is between 5% and 50%, for Deuk Laser Disc Repair, it’s 0%. Over 2,000 patients have been treated with this procedure, and there’s been a 99.6% success rate and zero complications.
To get started, Deuk Laser Disc Repair requires a very small incision, less than a quarter-inch long. A cylindrical rod called a dilator is inserted in the small opening to gently spread the muscle to create a small passage and guide through which the surgery is performed endoscopically.
The tip of the dilator is advanced into the symptomatic disc through the tear in the annulus where the herniation originates, and a tube called the retractor slides over the dilator and is carefully positioned into the painful disc. The rest of the entire Deuk Laser Disc Repair surgery will occur inside this narrow tube.
To access the spine, an endoscopic camera is inserted into the tubular retractor to allow the surgeon to guide the laser inside each symptomatic disc. This process ensures that bones and surrounding tissues are not damaged, unlike traditional spinal fusions, microdiscectomy, and artificial discs.
The Holmium YAG laser used in the Deuk Laser Disc Repair is manipulated accurately with millimeter precision under endoscopic visualization to remove only painful inflammatory tissue from the disc. The laser is precisely used to remove damaged disc material that is causing the pain.
The entire process takes about an hour and leaves less than one-quarter inch incision in the skin, which can be closed with a single stitch and a band-aid. After the surgery, the Deuk Laser Disc Repair patient is back home, enjoying life with a speedy recovery allowing normal activities without pain.
Another advantage of Deuk Laser Disc Repair is that no opioids or powerful narcotic painkillers are needed after surgery. If you’re suffering from chronic back and neck pain, submit your MRI for a free review with our team.
For most people with chronic back and neck pain, living with the pain is the biggest challenge they have ever faced. It can alter your daily habits, relationships, and career. Knowing how to alleviate some of your concerns and what to expect following therapy and surgery will assist you in making essential lifestyle changes.
The following are, therefore, general precautions to follow:
Abbott Spinal cord stimulators are ineffective in completely curing chronic back and neck pain; instead, they have been linked to complications such as spinal fluid leaks, headaches, and other complications.
While it can be recommended by pain management doctors, it may cause complications after several years of use. Mechanical failures, infections, and a general lack of efficacy can all contribute to this, necessitating additional procedures to maintain or reposition the device.
If you’re suffering from chronic back and neck pain, you should consider Deuk Laser Disc Repair. Deuk Laser Disc Repair is the most advanced laser spine surgery in the world, it doesn’t just treat your chronic pain, it cures it. This procedure is only offered at Deuk Spine Institute.
Deuk Spine Institute offers unique insight into the complexities of spinal health and nerves such as bulging discs, sciatica, spinal stenosis, herniated discs, and other conditions that cause severe chronic pain.
Contact us at Deuk Spine Institute today. We offer free consultation on your MRI scan. During a Deuk Spine MRI review, you will be asked to include both your actual MRI images (DICOM format strongly preferred) and the report.
Once this is done, Deuk Spine Institute and our team of doctors will review it and provide a customized plan on how to eliminate your pain once and for all.

